Patents and Other Intellectual Property Rights
CS MEDICA holds solid IPR protection, in a market with high barriers to entry, and a collaborative approach to innovation, while documenting efficacy and building trust through the Clinical Information management system (CIM) collecting post-marketing clinical test results in collaboration with distributors in local markets.
Patents
CS MEDICA strives towards granting patent acceptance on all present and future treatment products. All CS MEDICA’s treatment products (topical and oral products) as of today are patented in accordance with PCT (Patent Cooperation Treaty) covering 153 nations across the globe.
CS MEDICA currently has seven proceeding patents that are filed and pending.The patents pending are summarized below:
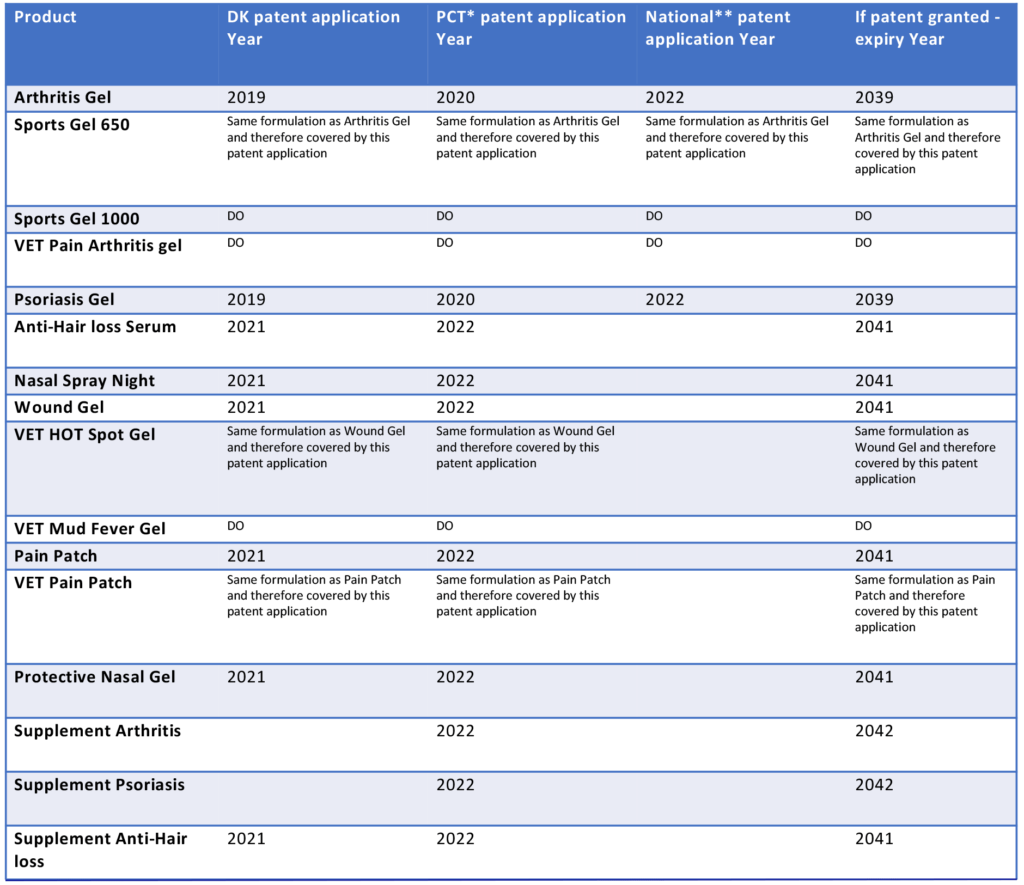
The patent applications includes the following phases:
Phase I (PRI): DK application (PRI)
Phase II (PCT): continuations of the DK application to the PCT application, covering 153 nations across the globe
Phase III (ORD): continuations of the PCT application to national applications
After the PCT (phase II), the patents will enter national phases (phase III) where CS MEDICA needs to determine in which countries and/or regions the Company intend to pursue patents. It is the current strategy to extend this protection in the US, China,, Japan, South Korea, Thailand, and Malaysia, Europe, Australia, New Zealand,, India, Canada, Israel, and South Korea.
The published patents of continuations of patent application can be found on the webpage patentscope.wipo.int by searching for “CS Medica and Henriksen” or via this link
The patents are intended to strengthen the protection of the Company’s products. If granted, the patents will protect the technology til 2039 (patent filed in 2019) and 2041 (patents filed in 2021).
Trademark
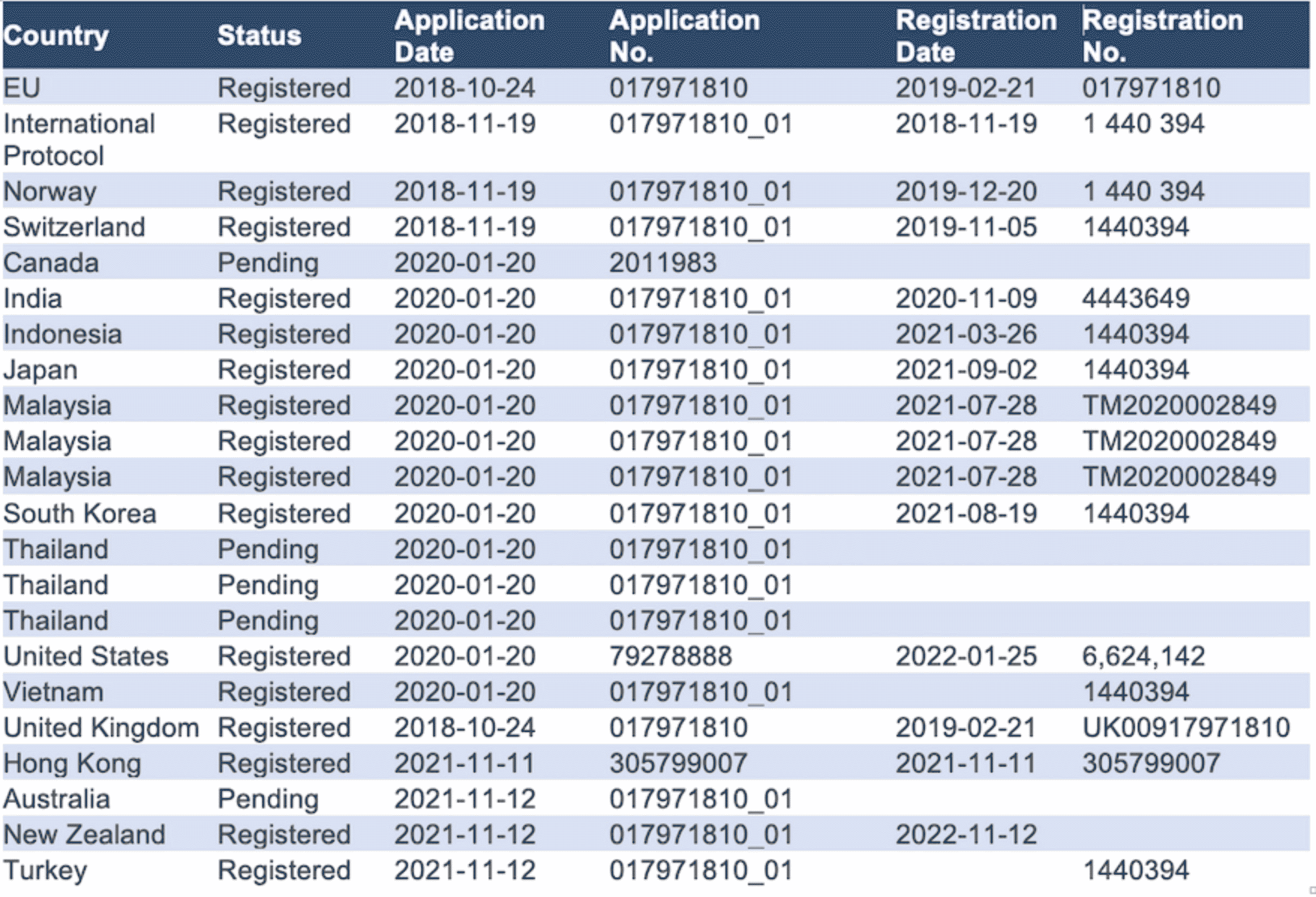
The Company protects its IPR by the mentioned patents and global trademarks registration in class 03, 05, and 10 – covering the following territories:
Digital Solutions
As a pioneer in the Cannabis market, we have stepped into a role of an active provider of relevant information and research data regarding cannabinoids and their properties to the wide public. We have developed digital solutions, which still are not common in the medical industry but crucial for our go-to-market strategy.
An Open Access Repository System dedicated to the investigation of cannabionoids and cannabionoids-based therapies is inaugurated at the brand site (www.cannasen.com). The OARS is an open-source system available to the public and is intended to provide and share all recent clinical test results, studies, journal publications and a variety of other educational resources within pain and autoimmune-related disorders. The OARS, currently covering 60 diseases, will be crucial for the research and development and in the implementation of the go-to-market strategy as a help the consumers to understand the benefits using cannabinoids in treatment. The OARS are keept up to date through monitoring online publications, where selected combinations of keywords are set up in a semantic market monitoring system (SMMS).
Together with our Product Information Portal (PIM) and Clinical Information Management system (CIM), great value is added to the collaboration between CS medica and our distributor. Both portals, available for us and our partners, will reduce manual workload, increase roll-out speed, and ensure the same level of quality to stakeholders in all locations. Moreover, the CIM will allow us as well as our distributors to perform post-marketing clinical trials in collaboration with local organizations, such as arthritis and psoriasis organizations. This facility ensures optimal local knowledge sharing via the organizations representing the factual disease profile.
The brandsite, www.cannasen.com, is currently available in English. As CS MEDICA penetrates new areas, they are supporting local distributors with the Company’s CANNASEN® store locator, linking to local online and physical stores.

